Abstract
CPX-351 is a dual-drug liposomal encapsulation of cytarabine and daunorubicin that is designed to achieve synergistic antileukemia activity. In a randomized, phase 3 study (NCT01696084) of adults aged 60-75 years with newly diagnosed, treatment-related AML or AML with myelodysplasia-related changes, CPX-351 demonstrated significantly improved overall survival (OS) and remission rates versus conventional cytarabine and daunorubicin (7+3 regimen), as well as a safety profile consistent with the known safety profile of 7+3 (Lancet JE, et al. ASCO 2016. Abstr 7000). To better understand the baseline characteristics that contributed to OS in this phase 3 study, a multivariate analysis was performed.
Patients were stratified by age (60-69 year or 70-75 years) and AML subtype (therapy-related AML, AML with antecedent myelodysplastic syndrome [MDS] with prior hypomethylating agents, AML with antecedent MDS without hypomethylating agents, AML with antecedent chronic myelomonocytic leukemia, or de novo AML with a MDS karyotype) and randomized 1:1 to receive 1 to 2 cycles of induction therapy with CPX-351 (100 units/m2 [100 mg/m2 cytarabine and 44 mg/m2 daunorubicin] on Days 1, 3, and 5 [Days 1 and 3 for second induction] or conventional 7+3 (cytarabine 100 mg/m2/day for 7 days [5 days for second induction] + daunorubicin 60 mg/m2 on Days 1-3 [Days 1-2 for second induction]). Patients with complete remission (CR) or CR with incomplete platelet or neutrophil recovery (CRi) could receive up to 2 consolidation cycles. Hematopoietic cell transplantation (HCT) was performed at the investigator's discretion.
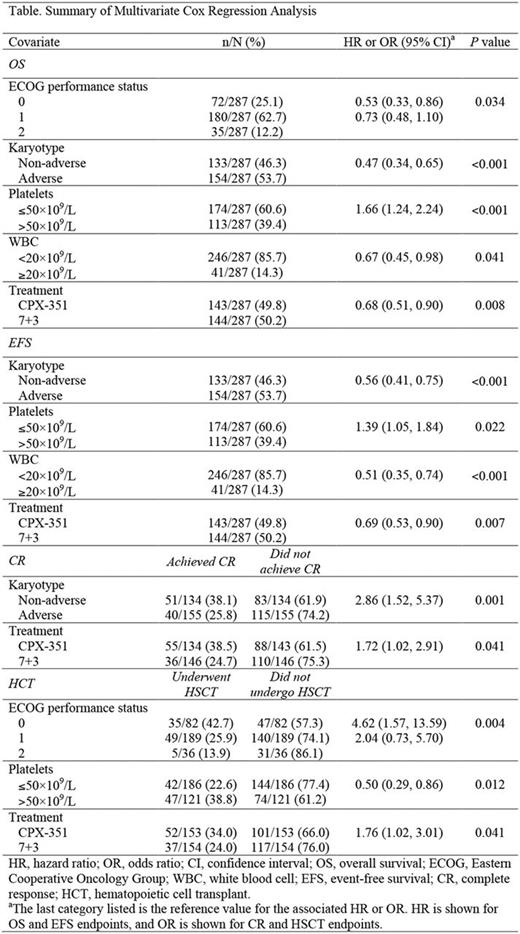
A multivariate analysis was conducted to evaluate the association of baseline covariates and efficacy endpoints (OS, event-free survival [EFS], best response of CR, and HCT). The evaluated categorical covariates included Eastern Cooperative Oncology Group (ECOG) performance status (0, 1, or 2), karyotype (adverse or non-adverse), white blood cell (WBC) counts (<20×109/L or ≥20×109/L), platelet counts (≤50×109/L or >50×109/L), hemoglobin levels (≤9 g/dL or >9 g/dL), bone marrow blast counts (<20%, 20%-40%, >40%-60%, or >60%), FLT3 -internal tandem duplication (ITD) status (positive or negative), and treatment arm (CPX-351 or 7+3). A Cox regression model was used for survival endpoints and a logistic regression model was used for response endpoints. Hazard or odds ratios and their associated 95% confidence intervals were calculated. A stepwise approach was used; covariates that met the statistical significance threshold of <0.05, using the Wald chi-square test, remained in the final model.
Detailed results for covariates identified as significant by the multivariate analysis are shown in the Table. The multivariate analysis indicated that lower ECOG performance status (P= 0.034), non-adverse karyotype (P <0.001), platelet count >50×109/L (P <0.001), WBC <20×109/L (P= 0.041), and treatment with CPX-351 (P= 0.008) all were significantly associated with improved OS. Non-adverse karyotype (P <0.001), platelet count >50×109/L (P= 0.022), WBC <20×109/L (P <0.001), and treatment with CPX-351 (P= 0.007) were associated with prolonged EFS. Non-adverse karyotype (P= 0.001) and treatment with CPX-351 (P= 0.041) were associated with a higher probability of achieving CR. A higher probability of progressing to HCT was associated with lower ECOG performance status (P= 0.004), platelet count >50×109/L (P= 0.012), and treatment with CPX-351 (P= 0.041). Hemoglobin levels, bone marrow blast counts, and baseline FLT3 -ITD status were not significantly associated with any of the evaluated endpoints.
The safety profile of CPX-351 was comparable to that of 7+3 in this study. The most common serious adverse events were febrile neutropenia (CPX-351, 8%; 7+3, 5%), respiratory failure (7%; 5%), ejection fraction decreased (6%; 6%), sepsis (8%; 3%), and pneumonia (7%; 4%). CPX-351 was associated with delayed neutrophil and platelet recovery compared with 7+3; however, early mortality rates were lower for patients receiving CPX-351 versus 7+3 at both 30 days (5.9% vs 10.6%, respectively) and 60 days (13.8% vs 21.2%).
In this multivariate analysis, after adjusting for covariates, treatment with CPX-351 was associated with improved efficacy across efficacy endpoints, consistent with analyses of individual endpoints previously described for this study.
Uy: GlycoMimetics: Consultancy; Novartis: Consultancy, Other: Travel Suppport; Boehringer Ingelheim: Consultancy. Lancet: Pfizer: Other: Institutional research funding; Biopath, Biosight, Boehringer Ingelheim, Celator/Jazz, Celgene, Janssen, Karyopharm Therapeutics, and Novartis: Consultancy. Cortes: ImmunoGen: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Teva: Research Funding; Sun Pharma: Research Funding; ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Lin: Jazz Pharmaceuticals: Consultancy. Ritchie: NS Pharma: Other: Research funding to my institution; Celgene: Consultancy, Other: Travel, Speakers Bureau; Pfizer: Consultancy, Other: Research funding to my institution; Bristol-Myers Squibb: Other: Research funding to my institution; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Other: Research funding to my institution, and travel, Speakers Bureau; Astellas Pharma: Other: Research funding to my institution. Stuart: Agios: Research Funding; MedImmune: Research Funding; Celator/Jazz: Research Funding; Bayer: Research Funding; Seattle Genetics: Research Funding; Sunesis: Consultancy, Honoraria, Other: Travel Support, Research Funding; Cantex: Research Funding; Novartis: Research Funding; Amgen: Consultancy, Honoraria; Incyte: Research Funding; ONO: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding. Strickland: Tolero Pharmaceuticals: Consultancy; Sunesis Pharamaceuticals: Consultancy, Research Funding; Baxalta: Consultancy; Boehringer Ingelheim: Consultancy; Daiichi Sankyo: Consultancy; Alexion Pharmaceuticals: Consultancy; Astellas Pharma: Honoraria; CTI BioPharma: Consultancy. Hogge: Novartis, Roche, and Sanofi: Consultancy. Stone: Novartis: Consultancy; Jazz,: Consultancy; Sumitomo Dainippon: Consultancy; Agios: Consultancy; Amgen: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy; Janssen: Consultancy; Cornerstone: Consultancy; Juno Therapeutics: Consultancy; Pfizer: Consultancy; Ono: Consultancy; Astellas: Consultancy; DSMN: Consultancy. Kolitz: Gilead, Magellan, and Novartis: Honoraria; Gilead, Novartis, and Seattle Genetics: Other: Travel Support; Boehringer Ingelheim, Cantex, Erytech, and Millennium: Research Funding; Gilead, Magellan, Novartis, Pharmacyclics, and Seattle Genetics: Consultancy; Celgene, Jazz: Equity Ownership. Schiller: Celator/Jazz: Research Funding. Wieduwilt: Sigma-Tau: Research Funding; Reata Pharmaceuticals: Equity Ownership. Chiarella: Celator/Jazz: Employment, Equity Ownership. Louie: Celator/Jazz: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal